Automated & Streamlined GMP Processes
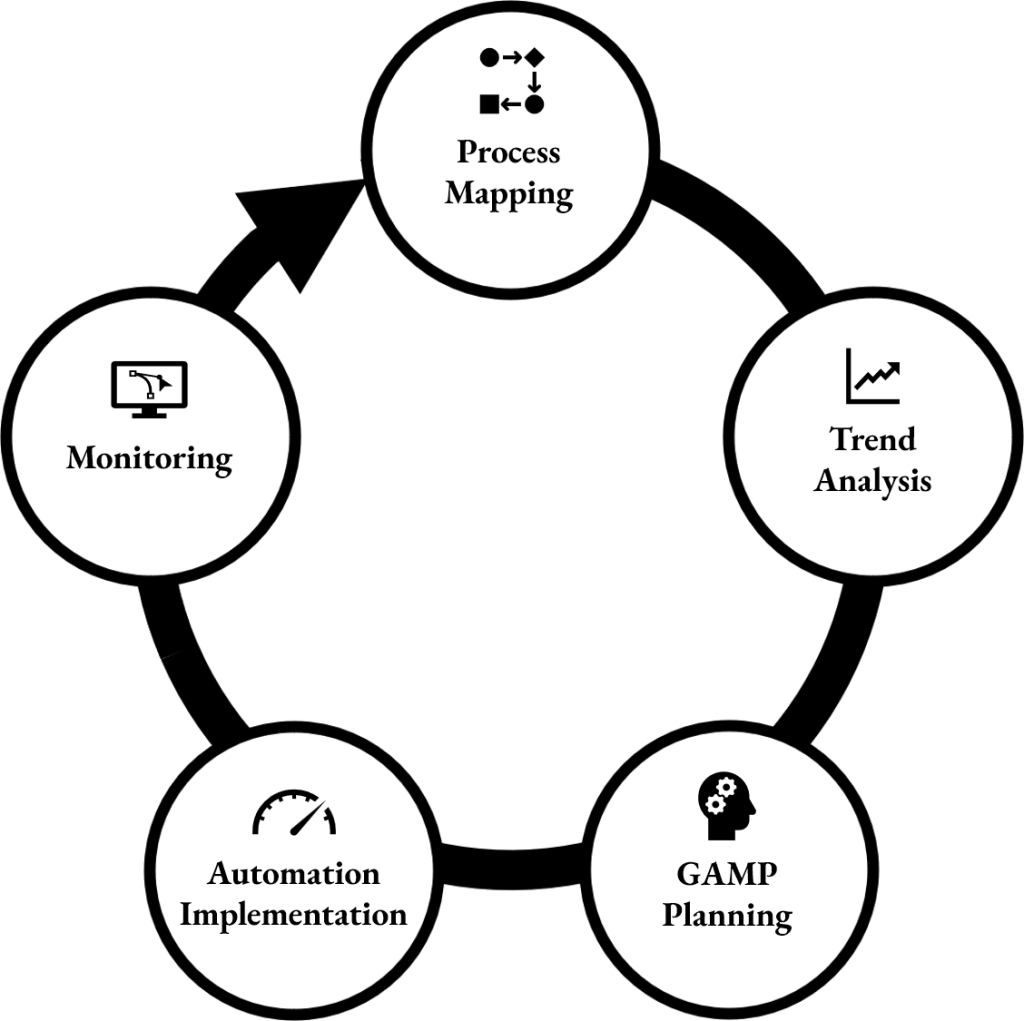
Stavigent identifies manual, error-prone steps in Good Manufacturing Practice (GMP) workflows and creates practical automation solutions to significantly reduce human errors. Using a structured approach that includes process mapping, deviation/Corrective and Preventive Action (CAPA) trend analysis, and risk-based Good Automated Manufacturing Practice (GAMP) aligned planning, the service provides clear automation opportunities and implementation guidance to enhance reliability, efficiency, and compliance.
This approach exceeds basic automation by employing a structured, GMP-focused method to precisely identify where manual handling, inconsistent execution, and documentation gaps elevate the risk of human error.
The process starts with detailed process mapping, documenting existing workflows, decision points, system interactions, and data flows to uncover hidden inefficiencies and high-risk handovers.
It involves analyzing deviations and CAPA trends through root-cause clustering, failure-mode patterns, and risk heat-mapping to determine which issues can be addressed through automation and which require training or procedural updates.
Using these insights, a risk-based automation plan is developed, aligned with GAMP 5 principles, defining clear requirements, impact levels, and validation expectations. This enables companies to understand which interventions are feasible, compliant, and high-value.
The result is a clear, actionable roadmap that enables GMP organizations to reduce deviations, improve process reliability, standardize documentation, and enhance operational compliance with minimal disruption.