GMP Documentation Development
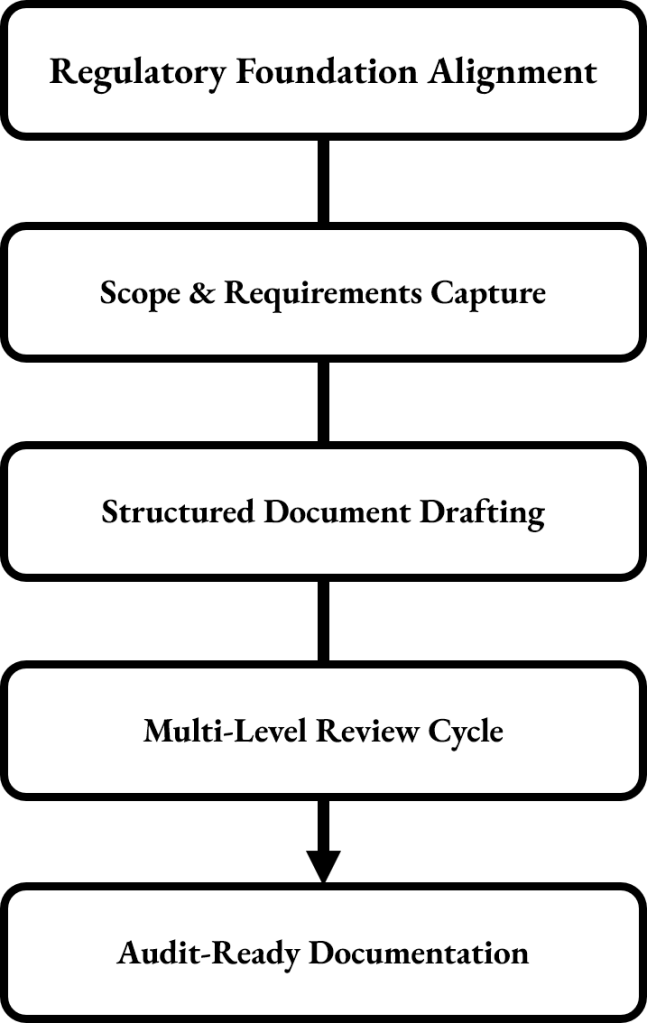
Stavigent provides audit-ready Validation and Quality Assurance (QA) documentation. Using a structured drafting and review process based on Good Manufacturing Practice (GMP), Annex 11, and Good Automated Manufacturing Practice (GAMP) 5 principles, documents are created with clarity, consistency, and regulatory compliance, ensuring companies get high-quality deliverables that integrate seamlessly into existing quality systems.
This method provides audit-ready Validation and QA documentation that easily integrates into existing Quality Management System (QMS). The service exceeds basic document creation by using a structured, regulatory-aligned approach that ensures clarity, traceability, and long-term compliance across all deliverables.
The process starts with a focused scoping and requirements phase, where existing documentation, systems, and intended use cases are examined. This involves understanding process criticality, system impact, data integrity needs, and regulatory expectations under GMP, GAMP 5, Annex 11, and FDA 21 CFR Part 11. This alignment guarantees that each document is based on a strong foundation of risk-based compliance.
Using structured drafting workflows, documents such as Validation Plans, User Requirement Specification (URS), Risk Assessments, Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols, Reports, Standard Operating Procedures (SOPs), and Data Integrity checklists are created with consistent logic, standardized terminology, and clear role responsibilities. Document structures adhere to GAMP 5 and Good Documentation Practices (GDP), reducing ambiguity and making it easier for inspectors to follow the reasoning and evidence trail.
Each document undergoes an internal quality review focused on traceability, completeness, and regulatory consistency. This involves cross-checking requirements against test evidence, verifying risk control measures, ensuring data integrity principles are embedded, and implementing document version control practices aligned with QMS expectations.
The result is a set of polished, compliant, and inspection-ready documents that facilitate smooth validation processes, withstand regulatory oversight, and lessen the workload on internal QA teams. Stavigent ensures that companies receive deliverables that are not only high-quality and approval-ready but also flexible, scalable, and aligned with current and future projects.