Standardized Documentation & Adaptive Templates
Stavigent consolidates scattered or outdated Good Manufacturing Practice (GMP) documents into a unified, compliant template system and enhances it with optional AI-supported drafting tools. Using gap assessments, template benchmarking, and a structured style guide approach, the service provides consistent, audit-ready templates that minimize variability, accelerate reviews, and help teams produce clear documents more efficiently with full control and compliance.
This service goes beyond just organizing documents by turning fragmented, inconsistent GMP documentation into a stable, controlled template system that teams can depend on. It aims to eliminate repetitive rewriting, reduce variability among authors, and ensure each new document begins with a compliant, audit-ready foundation.
The process starts with a comprehensive gap assessment of existing documentation to spot inconsistencies, missing sections, unclear instructions, and formatting issues across Standard Operating Procedures (SOPs), validation documents, protocols, risk assessments, and other GMP records. This review emphasizes where standardization is most needed and where templates can greatly reduce effort.
Industry benchmarking is then used to create a standardized template framework that incorporates best-practice standards from GMP, GAMP 5, ISO standards, and current regulatory trends. This involves developing a controlled style guide that covers terminology, numbering, risk statements, required sections, and wording conventions, ensuring that each document maintains consistent logic and structure.
To improve efficiency, the service provides modular templates that can be reused and easily updated over time. Instead of starting from scratch, teams only need to modify predefined sections relevant to a specific project, change, or system, which reduces drafting time, minimizes documentation errors, and accelerates review cycles.
For organizations interested in AI-supported documentation, optional AI-enhanced template builders are available. These tools enable users to automatically generate initial drafts of SOPs, User Requirement Specification (URS), protocols, or reports based on approved template structures, ensuring the output remains compliant, consistent, and fully traceable, while still giving the organization full control over the final content.
The result is a strong, easy-to-maintain documentation system that lowers workload, improves consistency, supports regulatory readiness, and enables teams to create high-quality GMP documents more quickly and confidently.
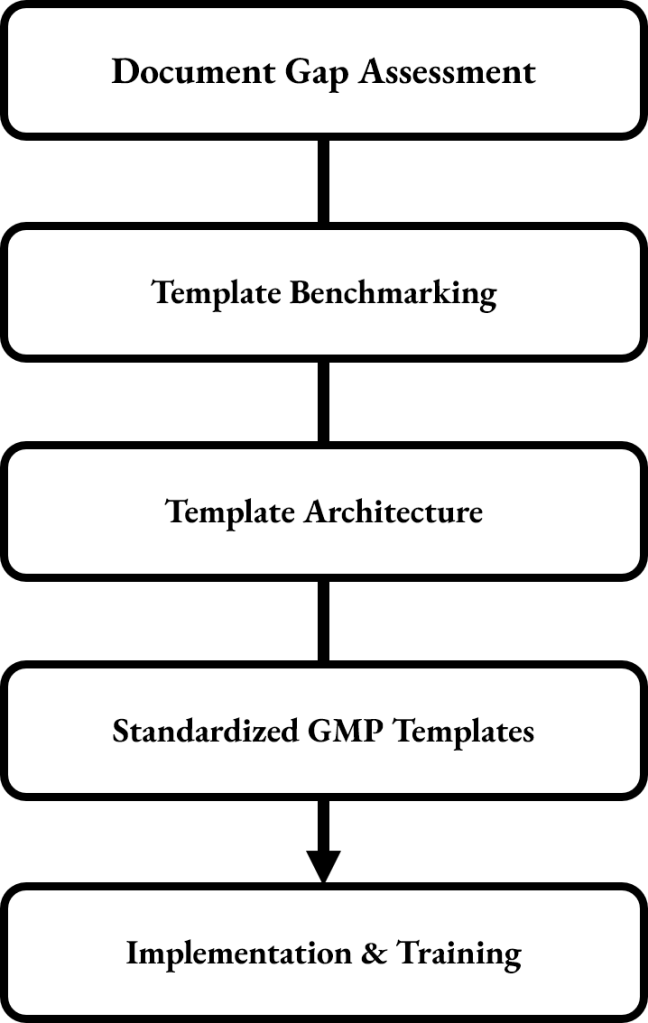
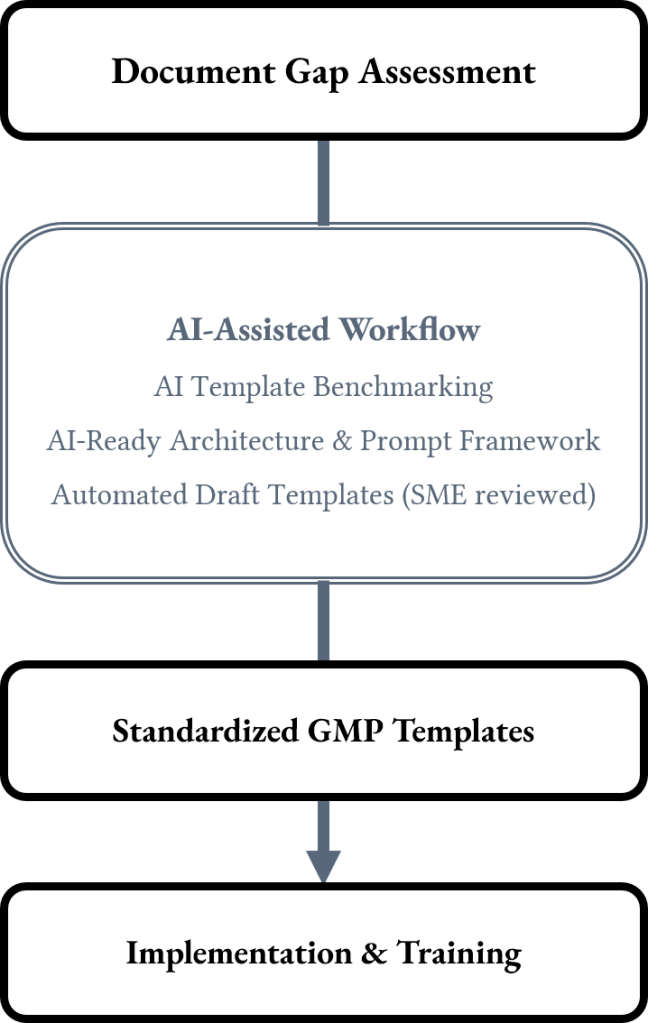
Standardization Workflow
